Robust 3-Dimensional visualization of human colon enteric nervous system without tissue sectioning
Details a new technique to image the enteric nervous system (ENS) and other cells that control motility in the human colon in three dimensions, by combining tissue clearing, immunohistochemistry, confocal microscopy, and quantitative image analysis.
Dataset Overview
Study Purpose: Current understanding and management of bowel motility disorders is limited by histologic techniques that rarely yield valuable information about cells that control motility. These cells are deep in the bowel wall, not uniform in distribution, and impossible to see in sufficient detail in small two dimensional sections routinely used for human pathology. The goal of the study was to develop an efficient and reproducible method to make human colon translucent, stain with antibodies, and visualize cells that control bowel motility in three dimensions.
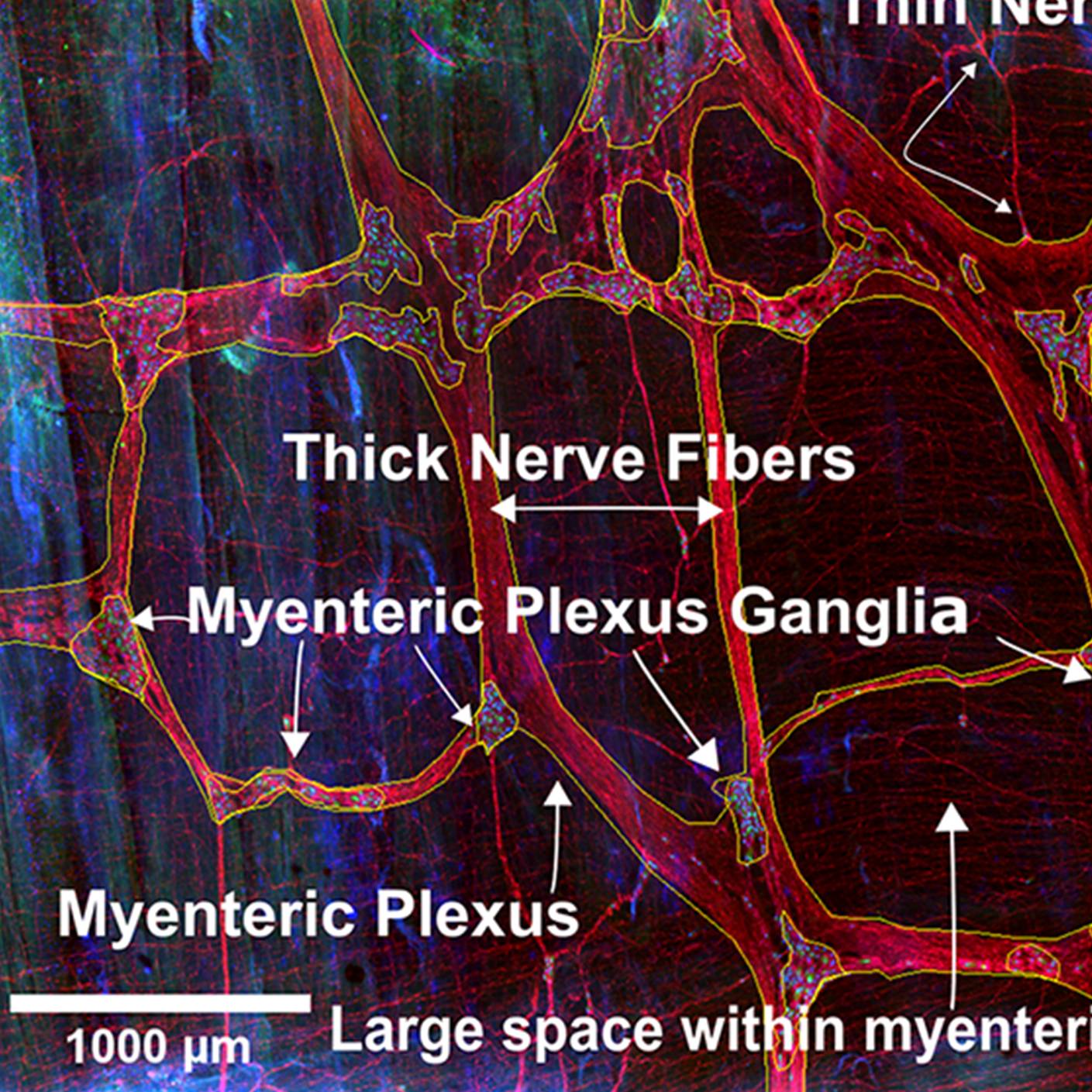
Data Collection: A new technique was developed to image the enteric nervous system (ENS) and other cells that control motility in the human colon in three dimensions, by combining tissue clearing, immunohistochemistry, confocal microscopy, and quantitative image analysis. The methods do not require tissue sectioning and can be scaled to evaluate cm2 bowel regions. Using human adult colon, one of the thickest bowel regions, many approaches and dozens of antibodies were tested until a method that worked consistently to visualize the human ENS in three dimensions was established. By imaging colon from people who do not have bowel motility disorders, unprecedented images were generated, and detailed quantitative data about the density of neurons and glia, ratios of cholinergic and nitrergic neurons, and organization of the ENS are provided. Murine images are shown for comparison.
Primary Conclusion: Most of what we need to know about ENS structure is only visible in three dimensions. We established an efficient and reproducible method for clearing, staining with antibodies, and imaging colon tissue. This approach works well to generate three-dimensional images of stained cells. We can visualize many cell types that control bowel motility, but the approach will also probably work to see other cell types with appropriate antibodies. Only a subset of antibodies works with this organic solvent-based fixation and clearing method.
Curator's Notes
Experimental Design: Fresh tissue from human colon obtained during surgery was cleaned, trimmed to remove fat, pinned flat, gently stretched, and then fixed in 4% paraformaldehyde before storage. To begin clearing, tissue is treated with 100% methanol, then permeabilized with Dent's bleach (Methanol, DMSO, and hydrogen peroxide) and rinsed with PBS. Blocking is performed for 3 days at room temperature. Incubation with primary antibodies occurs for 14 days at 37 oC. The unbound primary antibody is washed out over the course of one day. Secondary antibody staining is performed for three days at 37 oC. After washing in PBS, the tissue is dehydrated using a graded methanol series and then cleared using Murray's Clear (Benzyl Benzoate: Benzyl Alcohol). The method allowed visualization of many cell types that regulate bowel motility after staining with antibodies to HuC/D, VAChT, calretinin, S100B, PHOX2B, nNOS, CHAT, cKit, NFM, or neuron-specific beta 3 tubulin. For confocal imaging, the tissue is mounted in Murray's Clear.
Completeness: This study is complete.
Subjects & Samples: Male and female human colon tissue was used for this study, ages 2 months - 80 years old.
Primary vs derivative data: The primary folder is divided by subject identification. This folder contains tiff formatted confocal Z-stacks for each subject. No derivative folder exists.
Important Notes: (1) Antibody name and dilutions used for these studies are provided in the primary data manifest and the docs folder. (2) Docs folder exists for this study. This folder contains PDF formatted documents with information about all antibodies used for these studies.
Files
0 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Is Supplemented by
Heuckeroth, R., Huerta Lopez, S., Graham, K., & Sengupta, R. (2019). Human colon tissue clearing and Immunohistochemistry v1. https://doi.org/10.17504/protocols.io.wyeffte
