Safety testing of the Fecobionics device
We are developing a novel wireless device named Fecobionics for mapping colonic and anorectal neuromuscular function. The data of control device has been submitted. The current dataset contains results from the Fecobionics group of the safety study.
Dataset Overview
Study Purpose: We are developing a novel wireless device named Fecobionics for mapping colonic and anorectal neuromuscular function. The purpose of this study is to demonstrate the safety and performance of the device in vivo.
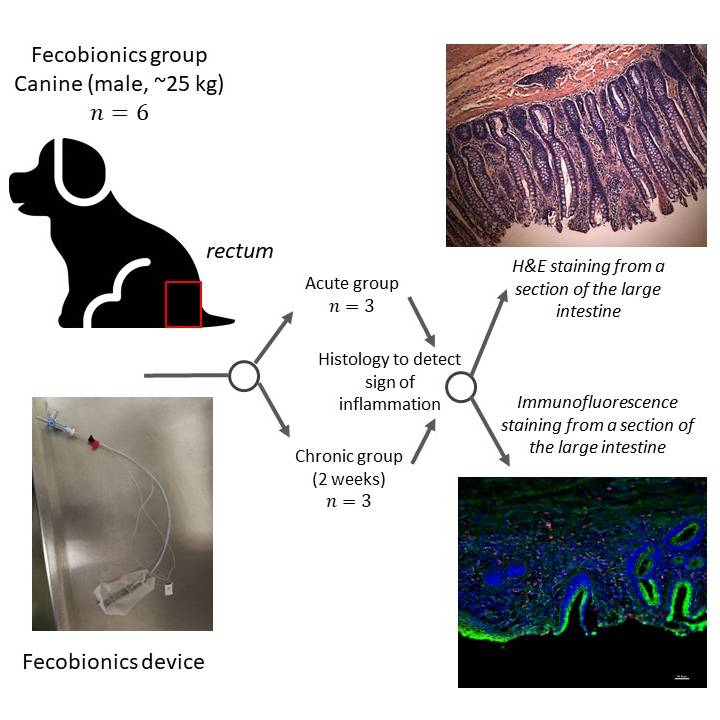
Data Collection: This dataset contains animal recording sheets and histology images from the Fecobionics group of our safety study in the canine lower GI tract. Hematoxylin and Eosin (H&E) and immunofluorescence (macrophages and caspase-3) staining were performed to identify inflammation in the tissue from different sections of the colon and anorectum.
Primary Conclusion: All procedures were performed successfully. All animals showed normal food intake and behavior. When compared to the control group, no obvious signs of inflammation were shown in both acute and chronic groups.
Curator's Notes
Experimental Design: Dogs after being anesthetized had the Fecobionics device transanally inserted into the rectum, then inflated at 20 ml, and then extracted manually or defecated naturally. The dogs were divided into two groups: The acute group in which the animals were euthanized immediately after the removal of the device, and the chronic group in which the animals were terminated after 2 weeks (N=3 of each). Tissue from different sections of the colon and anorectum were collected after the animals were euthanized. HE and immunofluorescence (macrophages and caspase-3) staining were performed to identify inflammation.
Completeness: This dataset is a part of the larger study: "Safety Study of Wireless Fecobionics Device" related dataset: https://doi.org/10.26275/dhbx-w17y
Subjects & Samples: Six male Mongrel dogs weighing about 25 kg, age 6-8 months were used in the study.
Primary vs derivative data: Data in the primary folder is divided by the subject identification. Each subject folder contains a pdf file with a detailed data collection sheet and sample folder with images of rectum and anus tissue sections stained with: 1). Hematoxylin and Eosin (H&E) Staining 10X microscopic images; 2). expressions of macrophage visualized with Anti-macrophage (red), mucosa staining (green), nuclear counterstain (blue) 20X microscopic images; 3). Expression of caspase-3 visualized with Anti-caspase3 (red), mucosa staining (green), nuclear counterstain (blue) 20X microscopic images.
Image data in the derivative folder (JPEG2000 and OME-TIFF) was derived from primary images (.tif). Each pair of images were merged into multichannel channel images derived from TIFs for each channel (red, blue, and green found in the SOURCE folder).
Tif images were converted with no compression to JPEG2000 (.jp2) by MBF Bioscience for web streaming and visualization on the SPARC Data Portal. Tif images were also converted with lossless compression to OME-TIFF (.tif) by MBF Bioscience. Hematoxylin and eosin image data (JPEG2000 and OME-TIFF) was derived from primary images (.JPG). JPEG images were converted with 20:1 compression to JPEG2000 (.jp2) by MBF Bioscience for web streaming and visualization on the SPARC Data Portal. JPEG images were also converted with lossless compression to OME-TIFF (.tif) by MBF Bioscience.
Files
0 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Is Supplemented by
Wang, Y., Kassab, G., Gregersen, H., & Bhavesh, P. (2019). Safety Study of Wireless Fecobionics Device v1. https://doi.org/10.17504/protocols.io.9nih5ce
