Transduction of systemically administered adeno-associated virus in the colonic enteric nervous system of adult mice
This dataset contains photomicrographs of the systemic AAV mouse colon enteric nervous system (ENS) that were acquired using confocal microscopes, visualized in three dimensions, and digitally segmented.
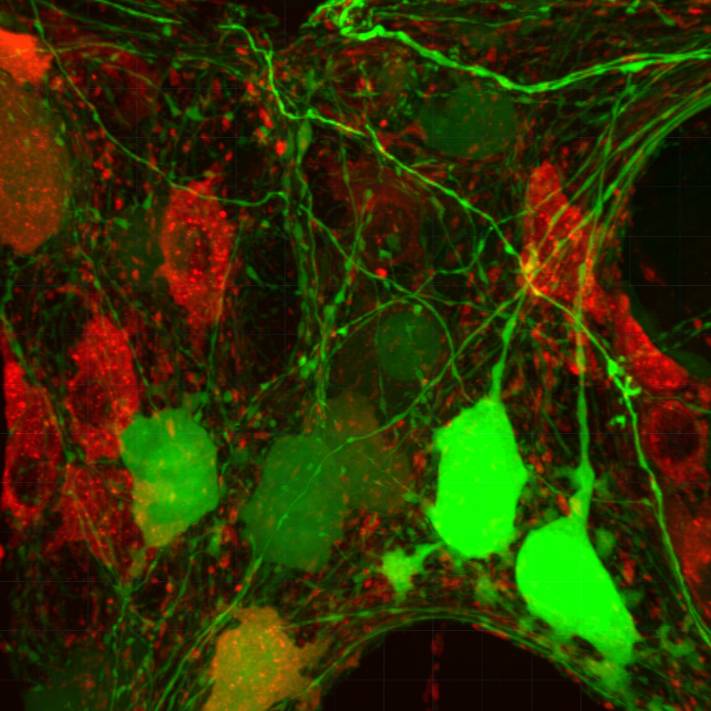
Dataset Overview
Study Purpose: To map and characterize the distribution patterns, segmental differences, morphology, and neurochemical identity of transduced cells in the colon of adult mice following systemic administration of adeno-associated virus (AAV)9 variants.
Data Collection: Photomicrographs were acquired in confocal microscopes, visualized in three dimensions, and segmented digitally.
Primary Conclusion: The data include photomicrographs demonstrating the segmental and regional differences of neurons and nerve fibers transduced by systemic delivery of AAV9 and AAV-PHP.S-tdTf in the adult mouse colon. The segmental difference with a decrease in the transduction of enteric neurons and nerve fibers in the transverse colon and almost none in the distal colon. In the proximal colon, the AAV9-transduced myenteric neurons were unevenly distributed in the myenteric plexus. The transduction was higher in cholinergic (ChAT) than inhibitory (nNOS) or sensory (calbindin) myenteric neurons, while there was no transduction in enteric glia that were located among the AAV9 and/or AAV-PHP.S-tdTf transduced neurons and nerve fibers. Iba1 positive macrophages with various morphologies did not show increase in different layers. AAV9/c-Kit data can be found in a published manuscript and a metadata set on SPARC Science portal.
Curator's Notes
Experimental Design: A multicolor adeno-associated virus system, including AAV9-CAG-GFP (AAV9), AAV-PHP.S-hSyn1-tdTomato farnesylated (PHP.S-tdTf), and other variants, was employed to investigate the segmental distribution, morphologies, and neurochemical coding of AAV transduction. Retro-orbital injections of the vectors were administered to male and female adult C57BL/6J, ChAT-Cre, and NOS1-Cre mice. After a 3-week survival period, euthanasia, and perfusion, gastrointestinal (GI) tissues and ganglia were prepared, with specific attention given to preserving layers in the colon using the Passive Clarity Technique (PACT). Colon tissue was prepared for microscopy with or without immunohistochemistry for neuronal and non-neuronal markers, including pan-neuronal markers such as HuC/D, protein gene product (PGP) 9.5, and NeuN. Additionally, markers for the most representative excitatory and inhibitory motor and sensory neurotransmitters, such as choline acetyltransferase (ChAT), neuronal nitric oxide synthase (nNOS), and calbindin, were assessed. Nerve fibers were examined for calcitonin gene-related peptide alpha (αCGRP), tyrosine hydroxylase (TH), and vasoactive intestinal peptide (VIP). Glial cells were investigated for glial fibrillary acidic protein (GFAP) and S100 calcium-binding protein B (S100B), while macrophages were assessed using ionized calcium-binding adaptor molecule 1 (Iba1). The interstitial cells of Cajal (ICC) were marked with c-Kit (a proto-oncogene encoding the receptor tyrosine kinase protein, also known as CD117). Photomicrographs of labeled tissues were acquired using a confocal microscope, visualized in three dimensions, and digitally segmented.
Completeness: This dataset is complete.
Subjects & Samples: 7-10 weeks old male (n=33) and female (n=15) wild (RRID:IMSR_JAX:000664 ) and transgenic (RRID:IMSR_JAX:028861) mice were used in this study.
Primary vs derivative data: The raw data in the Primary folder are organized by the subject ID; subsequently, the sample ID consists of original confocal microscopic images either as .lsm or .czi files. Image data (JPEG2000 and OME-TIFF) was derived from primary images (.lsm). Primary images converted with 20:1 compression to JPEG2000 (.jpx) by MBF Bioscience for web streaming and visualization on the SPARC Data Portal, 2 images per subject chosen for webstreaming. Primary images were also converted with lossless compression to OME-TIFF (.tif) by MBF Bioscience.
Important Notes: Other related publications
- Manuscript “Transduction of systemically administered adeno-associated virus in the colonic enteric nervous system and c-Kit cells of adult mice”. (https://doi.org/10.3389/fnana.2022.884280)
- Dataset with images of AAV9/c-Kit on SPARC Sciences (https://doi.org/10.26275/cheq-otb2)
- Manuscript “Multicolor sparse viral labeling and 3D digital tracing of enteric plexus in mouse proximal colon using a novel adeno-associated virus capsid”. (https://doi.org/10.1111/nmo.14014)
Files
0 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Is Supplemented by
C. Challis, R., Ravindra Kumar, S., Y. Chan, K., Challis, C., Beadle, K., J. Jang, M., Min Kim, H., S. Rajendran, P., D. Tompkins, J., Shivkumar, K., E. Deverman, B., & Lab, G. (2019). Systemic AAV vectors for widespread and targeted gene delivery in rodents v1. https://doi.org/10.17504/protocols.io.84ahyse
Wang, L., Challis, C., Liang, H., Li, S., Fowlkes, C., Sullivan, A., SR, K., & Taché, Y. (2020). Multicolor adeno-associate virus labeling and 3D digital tracing of enteric plexus in mouse proximal colon v1. https://doi.org/10.17504/protocols.io.bqavmse6
Wang, L., Yuan, P.-Q., Liang, H., & Tache, Y. (2020). Immunofluorescent methods for antibody test in mouse colon v1. https://doi.org/10.17504/protocols.io.bqi2muge
Described by
Wang, L., Yuan, P.-Q., Challis, C., Ravindra Kumar, S., & Taché, Y. (2022). Transduction of Systemically Administered Adeno-Associated Virus in the Colonic Enteric Nervous System and c-Kit Cells of Adult Mice. Frontiers in Neuroanatomy, 16. https://doi.org/10.3389/fnana.2022.884280
