Multi-scale rabbit cardiac electrophysiology models
A computational workflow for integration and implementation of a reusable and reproducible rabbit cardiac multi-scale electrophysiology model. Caption: Illustration of ion channels and action potential propogation in cardiac tissue.
Dataset Overview
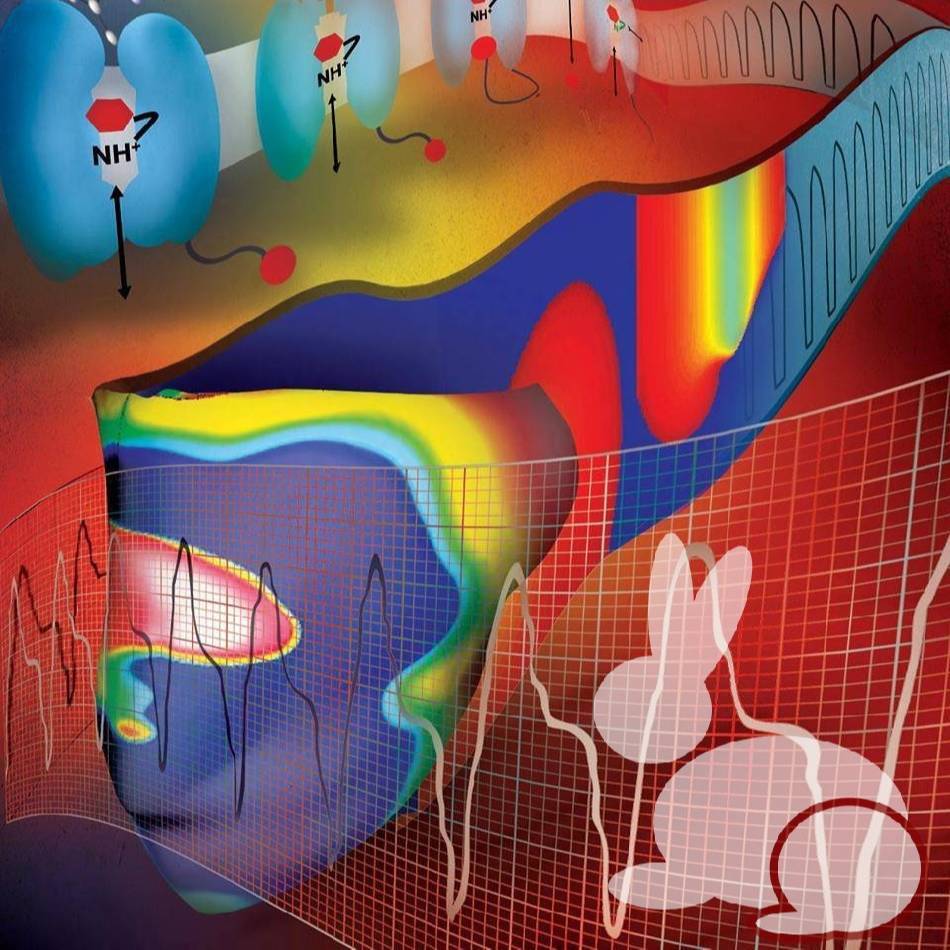
Study Purpose: A multi-layered model representation of the autonomic nervous system that includes sympathetic and parasympathetic branches, each with sparse random intralayer connectivity, synaptic dynamics, and conductance based integrate-and-fire neurons, generates firing patterns in agreement with experiment.
Data Collection: Not applicable – it is a computational study. This work is also based on previous research using Kepler flows to model cardiac physiology
Primary Conclusion: We present a multiscale model of autonomic control of cardiac electrophysiology that integrates data from the atomistic, subcellular, cellular and systems scale and predicts the effect of efferent stimulation of the sympathetic and parasympathetic branches of the autonomic nervous system on the cardiac sinoatrial node and ventricular myocardium.
Curator's Notes
Experimental Design: The workflow integrates multistep single-cell, 1D and 2D model simulations in a single automated process. The model formulation for ventricular cells from rabbits Soltis-Saucerman was implemented in the workflow. The model contains beta-adrenergic signaling pathways, and the firing rate model (unpublished Lewis model).
Completeness: This dataset is complete.
Subjects & Samples: This is a computational model dataset; thus no subjects are described.
Primary vs derivative data: Not applicable. This is a computational study.
Code Availability: A tutorial for this dataset can be found on o²S²PARC documentation here.
Files
1 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
References
Soltis, A. R., & Saucerman, J. J. (2010). Synergy between CaMKII Substrates and β-Adrenergic Signaling in Regulation of Cardiac Myocyte Ca2+ Handling. Biophysical Journal, 99(7), 2038–2047. https://doi.org/10.1016/j.bpj.2010.08.016
