Calcitonin gene-related peptide - immunoreactive (CGRP-IR) axon innervation of mouse stomach
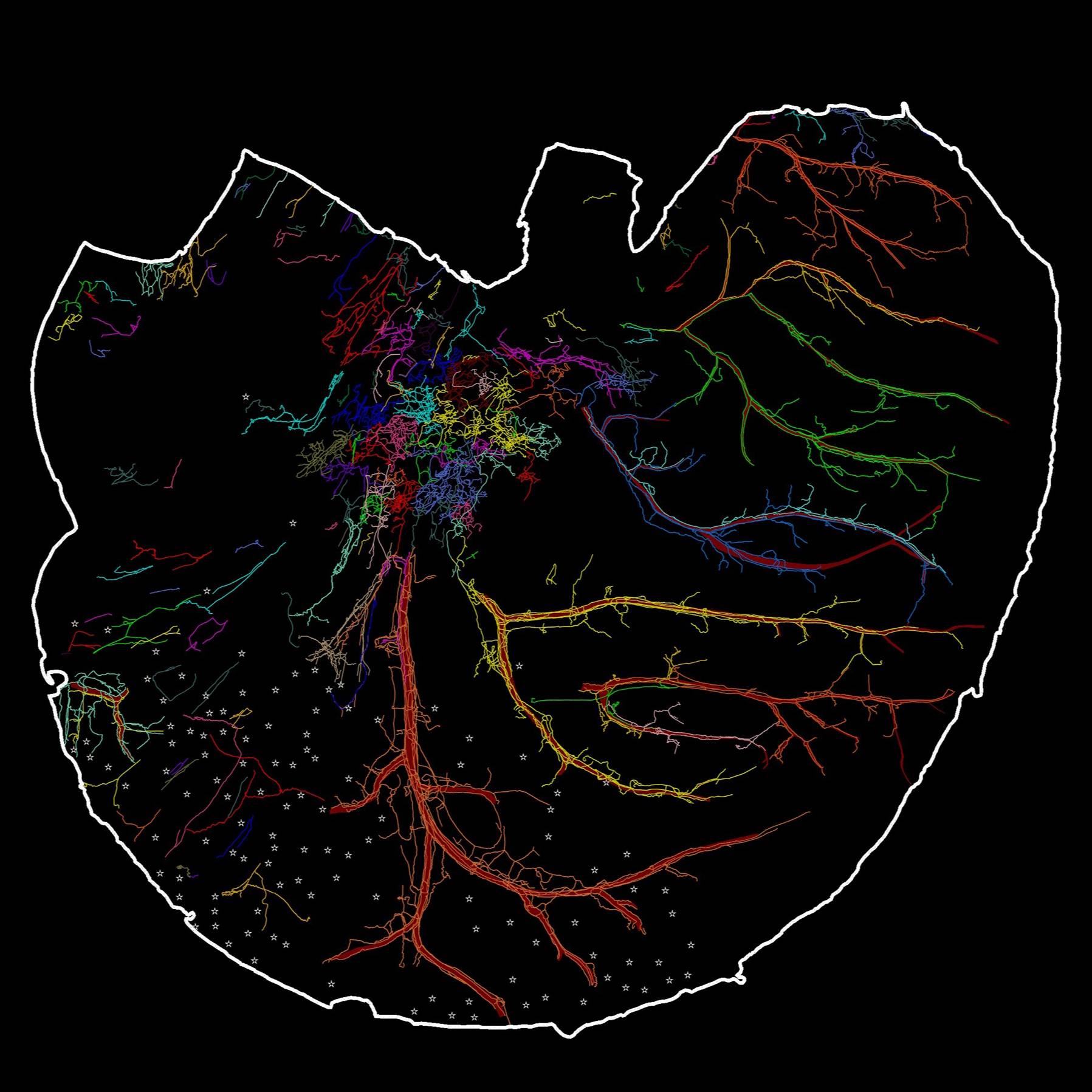
Immunohistochemistry revealed the morphology and distribution of nociceptor Calcitonin gene-related peptide (CGRP) immunoreactive in the muscular layers (longitudinal muscle, myenteric plexus, and circular muscle) of the mouse stomach.
Dataset Overview
Study Purpose: Nociceptive afferents innervate the stomach and send nociceptive signals centrally to the brain and locally to the enteric nervous system. Nociceptive afferents can be detected with a variety of different markers (e.g., CGRP, SP, TRPV1). However, the distribution and morphological structure of nociceptive axons and terminals have not yet been well determined in the flat-mounts of the whole stomach. In this study, we used calcitonin gene-related peptide (CGRP) as a marker to label nociceptive afferent axons and terminals in the in the flat mounts of the whole ventral and dorsal stomachs of mouse. This is the first time that we provided a topographical map of CGRP-IR innervation of the whole stomach at single cell/axon resolution. The work provides an anatomical foundation for functional studies of CGRP-IR axons in various regions of the stomach and their remodeling in diseases.
Data Collection: We applied a combination of state-of-the-art techniques, including confocal microscopy, Zeiss Imager microscopy, flat-mount tissue processing of the whole organ and immunohistochemistry, Neurolucida 360 tracing and integration of the tracing data into a 3D stomach scaffold to determine the distribution and morphology of CGRP-IR axons and terminals in the whole stomach. The animal model we used was C57BL/6J mouse. This dataset contains 2 components: 1) Montages of CGRP-IR axons and terminals in 7 male mouse stomachs (sub-dorsal-1, sub-dorsal-2,sub-dorsal-3, sub-dorsal-4, sub-ventral-1, sub-ventral-2, sub-ventral-3). The montage is a maximum projection image that is stitched from several hundreds of confocal single section images and is saved as .tif, .jpg, or .png. 2) The image corresponding to the stomach is digitized and traced using Neurolucida 360 and is saved as XML files.
Primary Conclusion: We found that 1) CGRP-IR axons formed extensive terminal networks in both ventral and dorsal stomachs. 2) CGRP-IR axons dramatically innervated the blood vessels. 3) In longitudinal and circular muscles, CGRP-IR varicose axons ran in parallel with the direction of the muscles. 4) CGRP-IR axons formed a complex network between the longitudinal and circular muscle layers. 5) In the myenteric ganglia, CGRP-IR axons formed varicose terminal contacts with individual myenteric neurons. 6) We did not observe any significant CGRP-IR myenteric neurons.
Curator's Notes
Experimental Design: Animals were euthanized with a lethal dose of sodium phenobarbital then perfused with at least 150 mL 40 °C phosphate-buffered saline via a blunt 18-ga needle inserted to the left ventricle, and blood was drained by cutting the inferior vena cava. The perfusion solution was switched to 150 mL ice-cold Zamboni’s fixative (15% picric acid, 2% paraformaldehyde in PBS, pH = 7.4) to fix the tissue. After the perfusion, the stomach was removed from the visceral cavity and trimmed to include the distal lower esophageal sphincter and the proximal pylorus. The stomach was post-fixed in the same fixative for 8 hours. After post-fixation, each part of the stomach was cleaned, and the mucosal and submucosal layer were dissected from the muscular wall of the stomach. The myenteric plexus together with the longitudinal muscle layer (facing the serosa of the stomach) and circular muscle layer (facing the submucosal layer) comprise the stomach muscular wall. The whole mount muscular wall of the stomach was processed with CGRP antibody (RRID:AB_1658411).
Completeness: This dataset is complete.
Subjects & Samples: Male C57BL/6J mice (RRID:IMSR_JAX:000664), n= 7, age 12-16 weeks, were used in this study.
Primary vs derivative data: Primary data folder is organized by the subject ID and sample ID subfolder containing .png confocal microscopy images of CGRP expressing neurons in the ventral and dorsal stomach along with .xml files containing 3D segmentation of those neuron tracings. Image data (JPEG2000 and OME-TIFF) was derived from primary images (.PNG). .PNG images were converted to standard tif using Fiji software. Output tif converted with 20:1 compression to JPEG2000 (.jpx) by MBF Bioscience for web streaming and visualization on the SPARC Data Portal. .TIF images were also converted with lossless compression to OME-TIFF (.tif) by MBF Bioscience.
Files
0 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Is Supplemented by
Nguyen, D., & Ma, J. (2021). Mapping CGRP-IR innervation of male mice stomach with Neurolucida 360 v1. https://doi.org/10.17504/protocols.io.bygmptu6
Nguyen, D., & Ma, J. (2022). Mapping CGRP-IR innervation of male mice stomach with Neurolucida 360 v1. https://doi.org/10.17504/protocols.io.6qpvr6k7zvmk/v1
