Optogenetic stimulation prevents lipopolysaccharide induced TNFa production
Animal study (C57BL/6J background transgenic mice) to determine the involvement of the superior mesenteric ganglion in the cholinergic anti-inflammatory pathway using optogenetic activation or blockade.
Dataset Overview
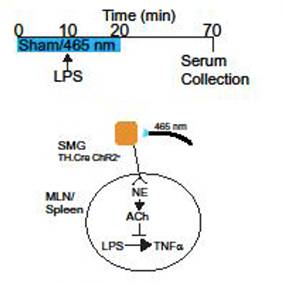
Study Purpose: This study explores the contribution of the superior mesenteric ganglion (SMG) in the efferent arm of the cholinergic anti-inflammatory pathway, mapping out neuronal connections between the vagal nerve and secondary lymphoid tissues including mesenteric lymph nodes (MLN) and the spleen.
Data Collection: Using conditionally expressed channelrhodopsin (CHAR2), we identified that selective stimulation of sympathetic post-ganglionic neurons in the superior mesenteric ganglion, induced NE (norepinephrine) and ACh (acetylcholine) release in MLN and spleen. Lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNFa) production was measured by ELISA (enzyme-linked immunosorbent assay).
Primary Conclusion: Release of ACh is dependent on Beta adrenergic receptors (B-AR) in vivo, as blockade with a B-AR selective antagonist prevents ACh release without affecting NE. Using selective optogenetic blockade, VNS-evoked release of NE and ACh in the spleen were blocked, indicating that this ganglion serves as junction in the relaying of neuronal signals to the spleen. Further highlighting the importance of the superior mesenteric ganglion, selective optogenetic stimulation was also able to recapitulate electrical VNS-mediated inhibition of lipopolysaccharide (LPS)-induced TNFa production. These studies identify the superior mesenteric ganglion as a critical node in the inhibition of immune cell function in the MLN and the spleen.
Curator's Notes
Experimental Design: The superior mesenteric ganglion was optogenetically stimulated multiple times at 250mA, 10Hz, 2ms. Blood serum was extracted via cardiac puncture prior to euthanasia. Post processing, TNFa was measured from the samples via an ELISA test.
Completeness: Complete
Subjects & Samples: This dataset was derived from a cohort of 29 male and female adult Cre-driver mice (6-8 weeks of age). Blood samples were collected for each mouse. There were three experimental groups: a control, a LPS stimulated group and a LPS+CHAR2 stimulated group.
Primary vs derivative data: The primary folder contains a csv file of the TNFa ELISA test results. There is no derived data folder but there is a helpful supplemental summary of the experiments in the protocol folder.
Code Availability: NA
Files
0 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Described by
Murray, K., Barboza, M., Rude, K. M., Brust-Mascher, I., & Reardon, C. (2019). Functional circuitry of neuro-immune communication in the mesenteric lymph node and spleen. Brain, Behavior, and Immunity, 82, 214–223. https://doi.org/10.1016/j.bbi.2019.08.188
Is Supplemented by
Murray, K., Sladek, J., & Reardon, C. (2019). Optogenetic Stimulation of superior mesenteric ganglion in a model of septic shock v1. https://doi.org/10.17504/protocols.io.wwbffan
