Using in vivo calcium imaging to examine joint neuron spontaneous activity and home cage analysis to monitor activity changes in mouse models of arthritis
This dataset encompasses studies on two mouse models of arthritis: antigen-induced arthritis and partial medial meniscectomy. It includes home cage activity analysis, joint histology, and in vivo calcium imaging.
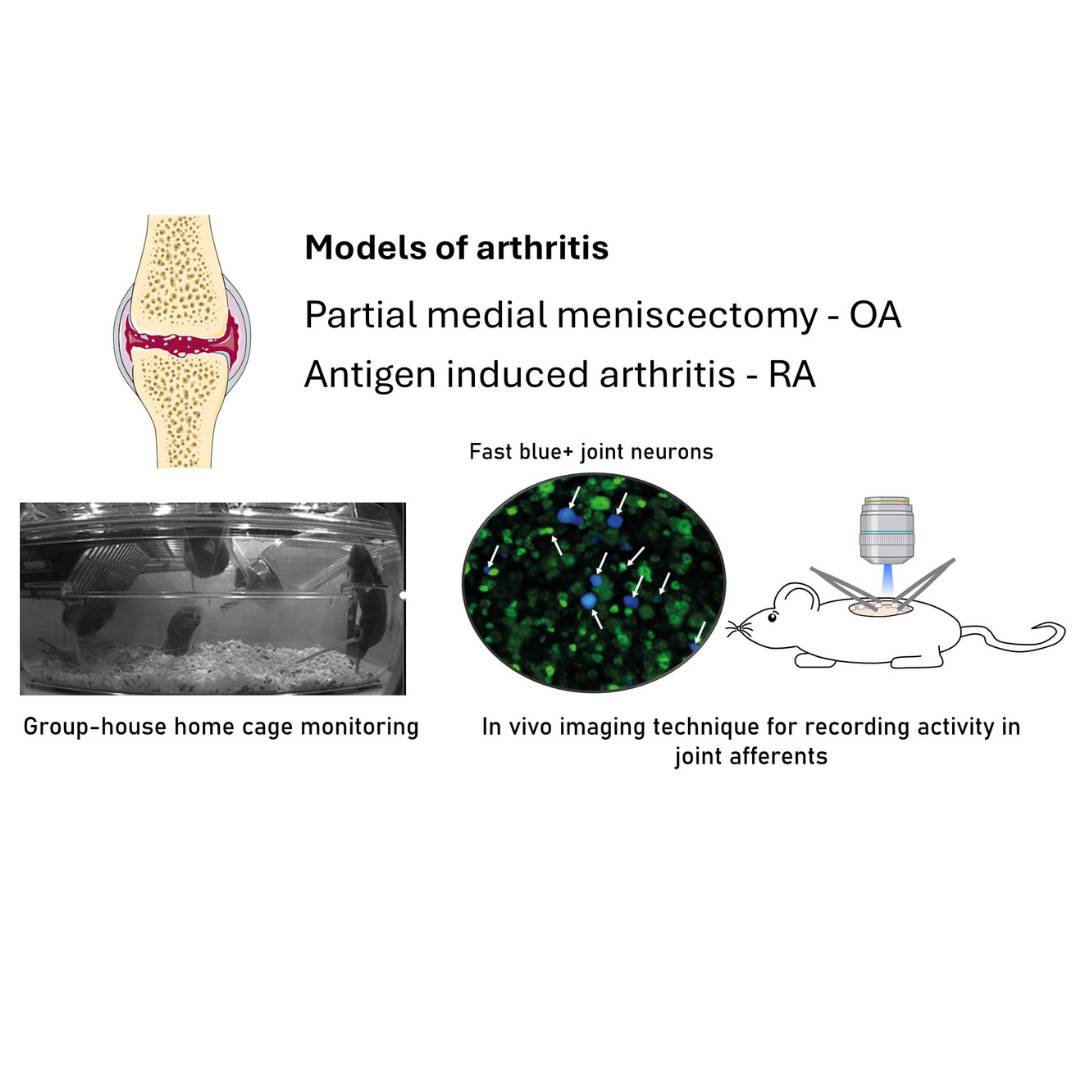
Dataset Overview
Study Purpose: Studying pain in rodent models of arthritis is challenging. For example, assessing functional changes in joints neurons is challenging due to their relative scarcity amongst all sensory neurons. Additionally, studying pain behaviors in rodent models of arthritis poses its own set of difficulties. Commonly used tests, such as static weight-bearing, often require restraint, which can induce stress and consequently alter nociception. The aim of this study was to evaluate two emerging techniques for investigating joint pain in mouse models of rheumatoid and osteoarthritis: In vivo calcium imaging to monitor joint afferent activity and group-housed home cage monitoring to assess pain-like behaviors. Specifically, we examined whether there was increased spontaneous activity in joint afferents and reduced locomotor activity following induction of arthritis.
Data Collection: Antigen induced arthritis (AIA) was used to model rheumatoid arthritis and partial medial meniscectomy (PMX) was used to model osteoarthritis. Group-housed home cage monitoring was used to assess locomotor behavior in all mice. In vivo, calcium imaging with GCaMP6s was used to monitor spontaneous activity in L4 ganglion joint neurons retrogradely labeled with fast blue 2 days following AIA and 13-15 weeks following PMX model induction. Cartilage degradation was assessed in knee joint sections stained with Safranin O and fast green in PMX mice.
Primary Conclusion: Group-housed home cage monitoring revealed locomotor changes in AIA mice, but not PMX mice (with n=10/group). In vivo calcium imaging can be used to assess activity in multiple retrogradely labeled joint afferents and revealed increased spontaneous activity in AIA but not PMX mice.
Curator's Notes
Experimental Design: Adult C57BL/6J mice were housed under standard conditions with food and water available ad libitum. experiments were conducted in compliance with the United Kingdom Home Office Animals (Scientific Procedures) Act (1986). Mice were randomly assigned to treatment and control groups for behavioral experiments, with assessments conducted by a blinded experimenter. To enable imaging of sensory neuron activity, the calcium indicator GCaMP6s was delivered via an adeno-associated viral vector (AAV9) injected subcutaneously into mouse pups. Following weaning, mice were housed with same-sex littermates until imaging was performed at 12 weeks post-injection. Retrograde labeling of knee joint afferents was performed using fast blue tracer under anesthesia. Surgical destabilization of the knee joint was achieved via partial meniscectomy, with sham surgery as a control. In a separate arthritis model, mice were immunized and later injected intra-articularly with mBSA. Behavioral assessments included static weight-bearing tests and home cage monitoring. In vivo imaging of sensory neurons was conducted under anesthesia, with the L4 dorsal root ganglion exposed and stabilized for confocal imaging of calcium dynamics. For the histology study, knee joints were fixed, decalcified, dehydrated, and embedded in paraffin after perfusion. Coronal sections were stained with hematoxylin and eosin, fast green, and Safranin O, then imaged in brightfield. Osteoarthritic changes were scored using the OARSI system by two blinded researchers.
Completeness: This dataset is complete.
Subjects & Samples: Male (n=25) and female (n=24) C57BL/6J mice (RRID:IMSR_JAX:000664), aged 24–26 weeks, were used in this study.
Primary vs derivative data: The dataset is organized into folders based on data type or modality: behavior, histology, and in-vivo-imaging. The in-vivo-imaging folder is structured by subject ID, with each subject folder containing in vivo calcium imaging data; detailed descriptions of each file can be found in the READ_ME.txt files within the respective folders. The behavior folder contains home cage analysis .csv files with all processed data, where each file represents a batch of n=4 mice. For Antigen-Induced Arthritis data, pre.csv files correspond to pre-antigen-induced arthritis challenge, while post.csv files correspond to post-challenge data. For the Partial Medial Meniscectomy model, each .csv file represents one batch at a specific time point. The Histology folder is organized by subject ID and sample ID, with each folder containing .czi files corresponding to slides of knee joint sections stained with Safranin O and Fast Green. Image data (.jp2 and .tif) in the derivative folder was generated from primary .czi images. Primary images were converted with 2:1 compression to JPEG2000 (.jp2) by MBF Bioscience for web streaming and visualization on the SPARC Data Portal. Additionally, primary images were converted with lossless compression to OME-TIFF (.tif) by MBF Bioscience.
Files
1 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Is Supplemented by
Goodwin, G., & Hobbs, C. (2024). Knee joint histology for evaluation of osteoarthrits in mice v1. https://doi.org/10.17504/protocols.io.6qpvr9oz3vmk/v1
Goodwin, G., & Denk, F. (2024). Group housed home cage monitoring for assessment of activity in mice v1. https://doi.org/10.17504/protocols.io.rm7vzk8e4vx1/v1
Goodwin, G., & Chisholm, K. (2024). In vivo calcium imaging of the L4 sensory ganglion in mice v1. https://doi.org/10.17504/protocols.io.eq2ly635rgx9/v1
Goodwin, G., Zarebska, J., & Vincent, T. (2024). Partial medial meniscectomy model of osteoarthrtis in mice v1. https://doi.org/10.17504/protocols.io.4r3l298rpv1y/v1
Described by
Goodwin, G. L., Marin, A.-C., Walker, J. V., Hobbs, C., & Denk, F. (2024). Using in vivo calcium imaging to examine joint neuron spontaneous activity and home cage analysis to monitor activity changes in mouse models of arthritis. https://doi.org/10.1101/2024.08.28.610097
