Neuronal mapping of human lung by immunostaining
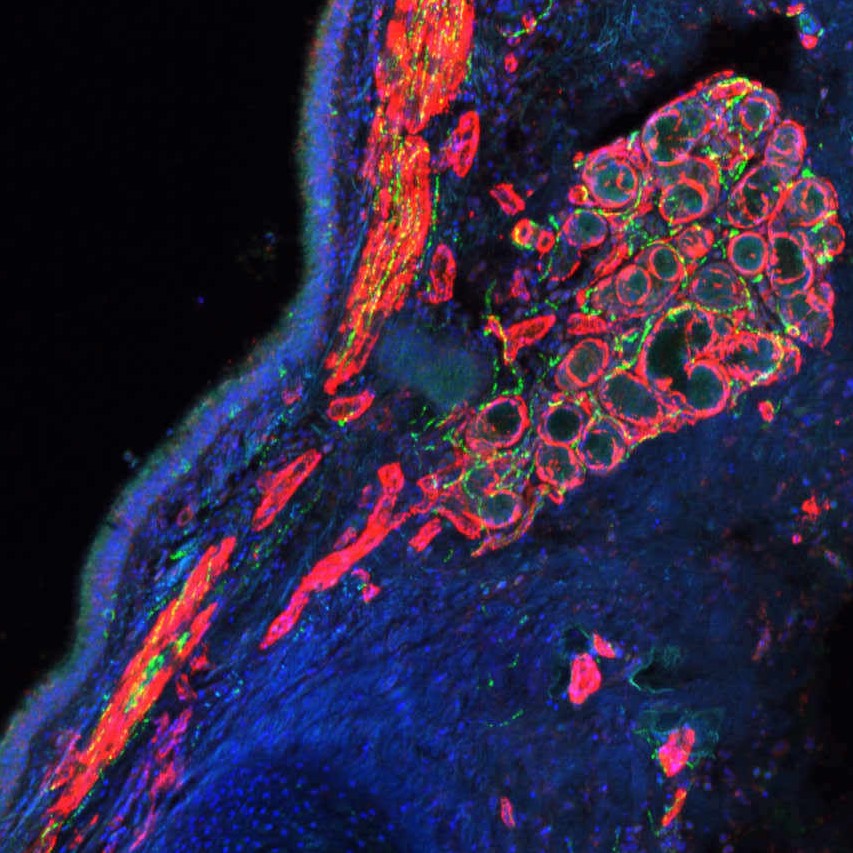
Immunofluorescence imaging of neuronal markers in human lung tissue
Dataset Overview
Study Purpose: To map the innervation pattern of the human lung in both normal and diseased lungs suffering from acute respiratory distress syndrome (ARDS).
Data Collection: This dataset contains confocal immunofluorescence imaging of neuronal markers in human lung tissue.
Conclusions: We used human adult normal lungs to test an array of neuronal markers for immunostaining. We found that pan-neuronal marker TUJ1 worked particularly well and was used as a positive control and for overall neuronal mapping. We noticed that most of the neuronal signal we saw was in the proximal airways of the lung and the extrapulmonary bronchus. Interestingly, unlike in mouse lungs, we saw no neuronal labeling in the distal lung samples that we examined. In the extrapulmonary and proximal airways, however, we saw significant labeling in the smooth muscle, along the airway epithelium, and in the submucosal glands. While the mouse only has submucosal glands in the very proximal trachea, the human lung has many more located throughout the extrapulmonary airway and within the lung, and we saw a significant amount of labeling in this structure. We found that the parasympathetic marker vesicular acetylcholine transporter (VAChT) worked well, labeling the smooth muscle and the parts of the submucosal gland. We saw a similar expression with the sympathetic marker Tyrosine Hydroxylase (TH) in both the smooth muscle and submucosal glands in addition to being near the vasculature. We next investigated how the innervation pattern may change in lungs suffering from acute respiratory distress syndrome (ARDS). We looked at samples from COVID-19 patients in addition to ARDS caused by an intracranial hemorrhage. The intracranial hemorrhage ARDS tissue was more difficult to obtain, and what we received was embedded in paraffin, so many antibodies did not give an adequate signal. TUJ1 staining was detected but looked reduced in the ARDS lungs compared to the control. We were able to obtain lung tissue from several COVID-19 patients through our connection with the LungMAP Human Tissue Core. The COVID-19 lungs presented technical challenges in getting clean immunostaining signals; there was a significant background for several antibodies that normally work well in control lung tissue. We noticed that all neuronal staining was very weak or non-existent in the COVID-19 lungs. This could be due to either the antibodies not working as well because of increased inflammation and immune infiltration or possibly due to an increase in cell death in these tissues. When we looked at extrapulmonary bronchus from COVID-19 patients, we were able to see staining of TUJ1, TH, and VAChT-positive neuronal fibers. The TH staining appeared weaker in the COVID-19 bronchus, but the TUJ1 and VAChT immunostainings looked comparable to control tissue. We hope that our findings here will serve as a useful reference for future studies of lung interaction with the nervous system.
Curator's Notes
Experimental Design: Lung samples were obtained from healthy donors (CONTROL) and from donors suffering from acute respiratory distress syndrome (ARDS) in addition to samples from COVID-19 patients (COVID). Lung tissue was fixed in 4%PFA and subjected to standard immunostaining protocol followed by confocal imaging.
Completeness: This dataset is part of a larger study: Foundational mapping of the neural circuits that control intrinsic lung function
Subjects & Samples: Samples from male and female human subjects (n=12) were used in this study.
Primary vs derivative data: The primary data is structured in folders, initially organized by subject ID and subsequently by sample ID subfolders. Within each sam- subfolder, you will find confocal images saved as maximum projection in .tif file format. There is no information about the magnification of the images taken. There is no derivative data folder.
Files
0 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Is Supplemented by
Barr, J., Verheyden, J., & Sun, X. (2021). Immunofluorescent staining of formalin-fixed OCT-embedded humanlung tissue v1. https://doi.org/10.17504/protocols.io.b2jnqcme
