3D segmentation of myelinated afferents of the rat bladder in the pelvic nerve
Using a combination of retrograde tracing from the bladder, an adeno-associated virus that preferentially labels myelinated afferents in rats, and a robust immunolabel for paranodes, myelinated bladder afferents were traced in the pelvic nerve of rats.
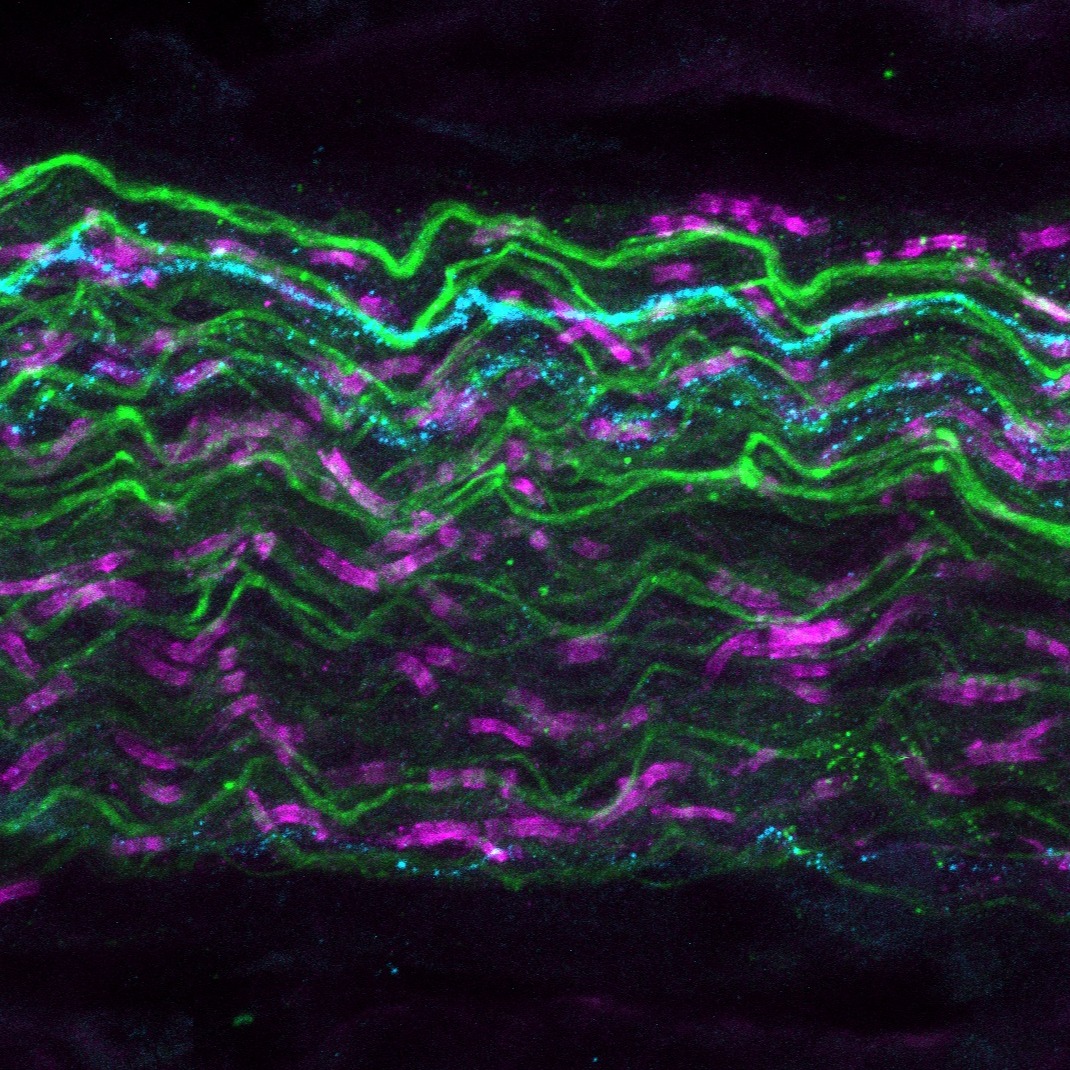
Dataset Overview
Study Purpose: Myelinated visceral afferents of the urinary bladder are critical for successful micturition. Most of these Aδ-class afferents reach the lumbosacral spinal cord via the pelvic nerves. Using a combination of retrograde tracing from the bladder (cholera toxin subunit B), and preferential sparse labeling of myelinated afferents (AAV-PHP.S), the myelinated afferents of the bladder could be visualized in Sprague-Dawley rats. Tracing these myelinated axons in Neurolucida 360, a rich dataset of myelinated bladder afferents was created, with nodes of Ranvier identified along their length using the paranode marker neurofascin.
Data Collection: This dataset contains wholemount image stacks of pelvic nerve fascicles from male and female Sprague-Dawley rats. These rats were intravenously injected with AAV-PHP.S, which preferentially transfects peripheral neurons, notably myelinated afferents in rats. In addition, the bladder was microinjected with cholera toxin subunit B (CTB) to retrogradely label bladder-innervating afferents. Major pelvic ganglia were removed and immunolabelled with pelvic nerve attached for AAV-PHP.S' fluorophore (TdTomato), CTB and neurofascin (paranode marker). The pelvic nerves were then sub-dissected from the major pelvic ganglia and fascicles containing AAV-PHP.S and CTB were imaged at 40x magnification on the LSM900 (Carl Zeiss Microscopes).
Primary Conclusion: The myelinated afferents innervating the bladder of the rat are thin in diameter, similar to that of A-delta class afferent fibers. Internode distance (i.e. length between nodes) varied greatly between axons and was unrelated to axon diameter.
Curator's Notes
Experimental Design: Male and female Sprague-Dawley rats were given tail vein injections of the sparse labeling adeno-associated virus with tropism for peripheral neurons, AAV-PHP.S (RRID:Addgene_104060). Nineteen days later, the retrograde tracer, cholera toxin subunit B (CTB), was microinjected into the wall of the urinary bladder. Two days after this procedure, the rats were anesthetized and perfused via the heart with heparinized saline, followed by buffered paraformaldehyde. Major pelvic ganglia (MPG) with attached pelvic nerves were then dissected and immunohistochemically labeled as whole mount preparations using antibodies for CTB, red fluorescence protein (part of the TdTomato in AAV-PHP.S), and neurofascin (a paranode marker). Pelvic nerve fascicles with CTB in AAV-PHP.S+ axons were subjected to large volume confocal microscopy at 40x magnification. In Neurolucida 360 (MBF Bioscience), axons that met three criteria were traced: CTB+, AAV-PHP.S+, and possessing paranodes.
Completeness: This dataset is complete.
Subjects & Samples: Male (n=3) and female (n=3) adult Sprague-Dawley (RRID:RRRC_00239) were used in this study.
Primary vs derivative data: The primary data is structured in folders, initially organized by subject ID and subsequently by sample ID subfolders. Within each sam- subfolder, you will find wholemount image stacks of pelvic nerve fascicles; for each fascicle, there is a .jpx image stack and a derivative .xml data file. These accompany each other, but can be opened separately in Neurolucida 360 and Neurolucida Explorer. Primary data images are converted to (JPEG2000 and OME-TIFF) formats for web streaming and visualization on the SPARC Data Portal. These converted images are stored in the derivative data folder.
Files
1 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Is Supplemented by
R Keast, J., B Osborne, P., & Wiedmann, N. (2019). Intracardiac perfusion with fixative for anatomical studies v1. https://doi.org/10.17504/protocols.io.bahzib76
Fuller-Jackson, J.-P., B Osborne, P., & R Keast, J. (2024). Axon tracing and node segmentation of myelinated bladder afferents in the pelvic nerve v1. https://doi.org/10.17504/protocols.io.bp2l62x3rgqe/v1
R Keast, J., B Osborne, P., & Wiedmann, N. (2024). Immunohistochemical staining of wholemount major pelvic ganglia (MPG) for analysis of myelinated bladder afferents v1. https://doi.org/10.17504/protocols.io.yxmvme3nbg3p/v1
R Keast, J., B Osborne, P., & Fuller-Jackson, J.-P. (2021). Use of cholera toxin subunit B to label neural projections to lower urinary tract organs v1. https://doi.org/10.17504/protocols.io.byqbpvsn
R Keast, J., Wiedmann, N., & B Osborne, P. (2021). Intravenous (tail vein) injection of AAV into rat v1. https://doi.org/10.17504/protocols.io.6qpvrdbbpgmk/v1
