Selective recording of physiologically evoked neural activity in a mixed autonomic nerve using a minimally invasive array
This data set includes recording and analysis of extracted signals form the pelvic nerve during cystometry testing in anesthetized rats.
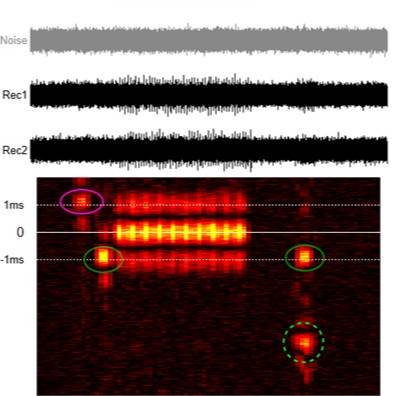
Dataset Overview
Study Purpose: Real-time closed-loop control of neuromodulation devices requires long-term monitoring of neural activity in the peripheral nervous system. Although many signal extraction methods exist, few are both clinically viable and designed for extracting small signals from fragile peripheral visceral nerves. Here we report that our minimally invasive recording and analysis technology extracts low to negative signal to noise ratio (SNR) neural activity from a visceral nerve with a high degree of specificity for fiber type and class.
Data Collection: Complex activity was recorded from the rat pelvic nerve that was physiologically evoked during controlled bladder filling and voiding, in an extensively characterized in vivo model that provided an excellent test bed to validate our technology. Urethane-anesthetized male rats (n = 12) were implanted with a 4-electrode planar array and the bladder instrumented for continuous-flow cystometry, which measures urodynamic function by recording bladder pressure changes during constant infusion of saline.
Primary Conclusion: We demonstrated that differential bipolar recordings and cross-correlation analyses extracts afferent and efferent activity and discriminated between subpopulations of fibers based on conduction velocity. Integrated Aδ afferent fiber activity correlated with bladder pressure during voiding (r2: 0.66 ± 0.06) and was not affected by activating nociceptive afferents with intravesical capsaicin (r2: 0.59 ± 0.14, P = 0.54, n = 3). Collectively, these results demonstrate our minimally invasive recording and analysis technology is selective in extracting mixed neural activity with low/negative SNR. Furthermore, integrated afferent activity reliably correlates with bladder pressure and is a promising first step in developing closed-loop technology for bladder control.
Curator's Notes
Experimental Design: Male rats were anesthetized with urethane, and a pelvic nerve array and bladder catheter were implanted (non-recovery, acute experiment). The aim was to assess the performance of the array in recording neural activity during cystometry testing (infusion of the bladder with saline). Infusion of various pharmacological drugs during cystometry testing was considered to modify the electrophysiological activity of different subclasses of neural populations in the pelvic nerve. The ability of the array to record neural activity from different subclasses (low mechanical threshold receptors, nociceptors, and autonomic efferents) was tested through these experiments. Verification of appropriate electrode placement on the nerve was ensured by recording electrically-evoked neural activity. Subsequently, the following tests were performed: 1) Cystometry (saline) at slow, medium, and fast flow rates; 2) Maintenance of bladder pressure for 2 minutes at approximately 75% of total filling capacity; 3) Insertion of a colonic balloon into the colon via the rectum, with the recording of activity during sustained colonic balloon pressures; 4) Induction of pharmacological manipulation by infusing 0.3 µM capsaicin or 2% lidocaine into the bladder.
Completeness: This dataset is complete.
Subjects & Samples: Male (n=12) adult rats were used in this study.
Primary vs derivative data: The primary data is structured by subject ID files include data collected from individual animals: a file(.h5) containing electrically evoked compound action potential waveforms. This file also contains code to allow visualization of cystometry traces and neural signals. A subfolder "Day 0" (.ccf, .nev, ns1, ns2) contains cystometry traces and corresponding neural signal data. There is no derivative data folder.
Important Notes: The article may be accessed via: https://doi.org/10.1063/5.0164951. All data in the publication is contained within these files. Manifest (.xlsx) containing details of all files and relevant information for interpretation of files
Files
1 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Described by
Is Supplemented by
B Fallon, J., & Payne, S. (2020). Electrophysiological recording of electrically-evoked compound action potentials v1. https://doi.org/10.17504/protocols.io.bfwyjpfw
B Fallon, J., Payne, S., R Keast, J., & B Osborne, P. (2020). Implantation of a pelvic nerve array in rats v1. https://doi.org/10.17504/protocols.io.bjvxkn7n
R Keast, J., B Osborne, P., & Wiedmann, N. (2019). Cystometry in awake rats v1. https://doi.org/10.17504/protocols.io.bakjicun
