Light microscopic analysis of synaptic input to neurons in the rat major pelvic ganglion
High resolution 3D confocal images of autonomic neurons and closely opposing synaptic boutons were segmented and analysed to obtain statistics on preganglionic input to immunohistochemically classified lower urinary tract projecting MPG neurons.
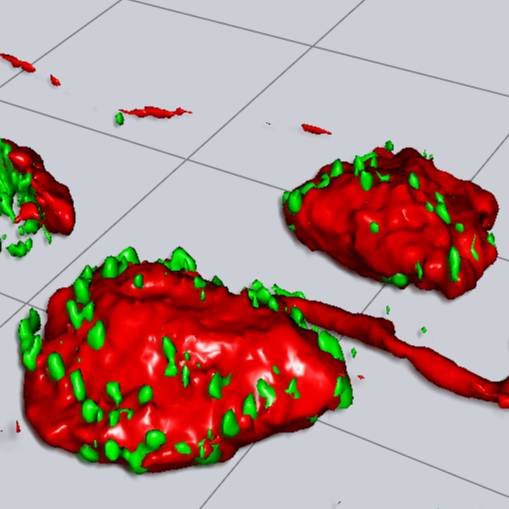
Dataset Overview
Study Purpose: In rodents, bilateral major pelvic ganglia (MPG) are the primary source of autonomic motor innervation of pelvic viscera: including the urinary bladder and urethra in the lower urinary tract (LUT); colorectal regions of the gastrointestinal tract; and the sexually dimorphic reproductive organs. Two classes of autonomic postganglionic neurons in the MPG are distinguished by the spinal cord origin of their input. Parasympathetic neurons receive input from autonomic preganglionic neurons in the sacral parasympathetic nucleus (SPN), which spans lumbosacral spinal cord segments (L6-S1 in rat) caudal to the lumbar enlargement. Sympathetic neurons receive input from preganglionic neurons in parts of the intermediolateral nucleus (IML) or dorsal commissural nucleus (DCN) that span the thoracolumbar segments (L1-L2) rostral to the lumbar enlargement. This dataset was acquired in the context of a study which aimed to determine if the anatomical organization of preganglionic synapses on different classes of MPG neurons is homogeneous or shows structural evidence of functional specialization.
Data Collection: The study aim was addressed by acquiring a large dataset of high resolution 3D confocal images of MPG neuronal cell bodies in which synaptophysin-immunofluorescence was used a surrogate marker of preganglionic synapses. Single neurons and surrounding synapses were then segmented using Imaris to produce 3D surface models of the neuron soma and associated synaptophysin+ volumes, which were used to measure morphological parameters in different classes of MPG neurons identified by their projection target in the lower urinary tract (LUT) or by immunofluorescence markers.
Primary Conclusion: The dataset provides evidence that autonomic preganglionic synaptic inputs to rat postganglionic MPG neurons show morphological specializations associated with the class of the postsynaptic neuron.
Curator's Notes
Experimental Design: The dataset was generated from multichannel 3D confocal images featuring 1292 major pelvic ganglion (MPG) neurons from adult male and female rats. To label lower urinary tract (LUT) projection neurons, fluorescent retrograde neural tracers (Fast blue or Fluorogold) were injected into the bladder body, bladder trigone, or urethra (proximal end) of anesthetized animals. Major pelvic ganglion (MPG) samples were collected, fixed in PFA, cryopreserved, and then sliced into thin sections (50µm) for subsequent analysis. Synaptic specializations were visualized and quantified in 69 female and 78 male bladder body+ neurons and 82 female and 84 male bladder trigone+ neurons. Similar analyses were performed in urethra+ neurons, further classified through immunohistochemistry using tyrosine hydroxylase (TH) and neuronal nitric oxide synthase (nNOS) antibodies. This led to the identification of 74 urethra+ and 70 urethra+/nNos+ female neurons, as well as 63 urethra+, 70 urethra+/nNos+, and 65 urethra+/TH+ male neurons. For comparison, immunohistochemistry was employed to identify groups of 196 nNOS+ female neurons, and 214 nNos+ and 227 TH+ male neurons in the MPG, in which projection targets were not identified. Regions of interest (ROIs) were imaged with a Zeiss 880 confocal microscope using Airyscan. The multichannel 3D image stacks were initially saved as Zeiss .czi files, imported, and converted to .ims files using Imaris. Within each image stack, one or more single neurons were classified (fluorescent neural tracer or immunofluorescence) and then segmented in Imaris to create surface models of the cell body of MPG ganglion neurons and their associated synaptic boutons. This was achieved through thresholding the fluorescence of either the neural tracer (Fluorogold or Fast Blue), cytoplasmic immunostaining for nNOS or TH, or marker of synaptic boutons. Morphological measurements extracted from the surface models in Imaris were exported and saved in .xlsx files using Excel.
Completeness: This dataset is complete.
Subjects & Samples: Female (n=17) and male (n=16) adult Sprague Dawley rats (RRID:MGI:5651135) were used in this study.
Primary vs derivative data: The primary data is structured in folders, initially organized by subject ID and subsequently by sample ID subfolders. Within each sam- subfolder, you will find 3D multichannel images containing one or more analyzed neurons and their surface models saved as .ims files. Additionally, each subfolder includes a set of morphological measurements for each analyzed neuron saved as .xlsx files. The derivatives folder contains all morphological measurements from the dataset saved in a .xlsx file. Primary data images are converted to (JPEG2000 and OME-TIFF) formats for web streaming and visualization on the SPARC Data Portal. These converted images are stored in the derivative data folder.
Important Notes: The documents folder contains a summary R notebook with a data dictionary saved as a .html file.
Files
0 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Is Supplemented by
R Keast, J., & B Osborne, P. (2019). Use of tracer dyes to label neural projections to lower urinary tract organs v1. https://doi.org/10.17504/protocols.io.w2xfgfn
R Keast, J., B Osborne, P., & Wiedmann, N. (2019). Intracardiac perfusion with fixative for anatomical studies v1. https://doi.org/10.17504/protocols.io.bahzib76
R Keast, J., & B Osborne, P. (2019). Immunohistochemical labeling of thick cryosections from pelvic ganglia v1. https://doi.org/10.17504/protocols.io.bakcicsw
R Keast, J., & B Osborne, P. (2019). Confocal microscopy and characterization of synaptic boutons associated with ganglion neurons v1. https://doi.org/10.17504/protocols.io.bakdics6
R Keast, J., & B Osborne, P. (2019). Light microscopic analysis of synaptic input to neurons in the rat major pelvic ganglion [keast-003] v1. https://doi.org/10.17504/protocols.io.bakiicue
