Selective stimulation of the ferret abdominal vagus nerve with multi‐contact nerve cuff electrodes
This dataset contains the effects of abdominal vagus nerve stimulation on nodose ganglion cell activity recorded from multi-electrode arrays and gastrointestinal myoelectric responses recorded with serosal surface electrodes in the ferret.
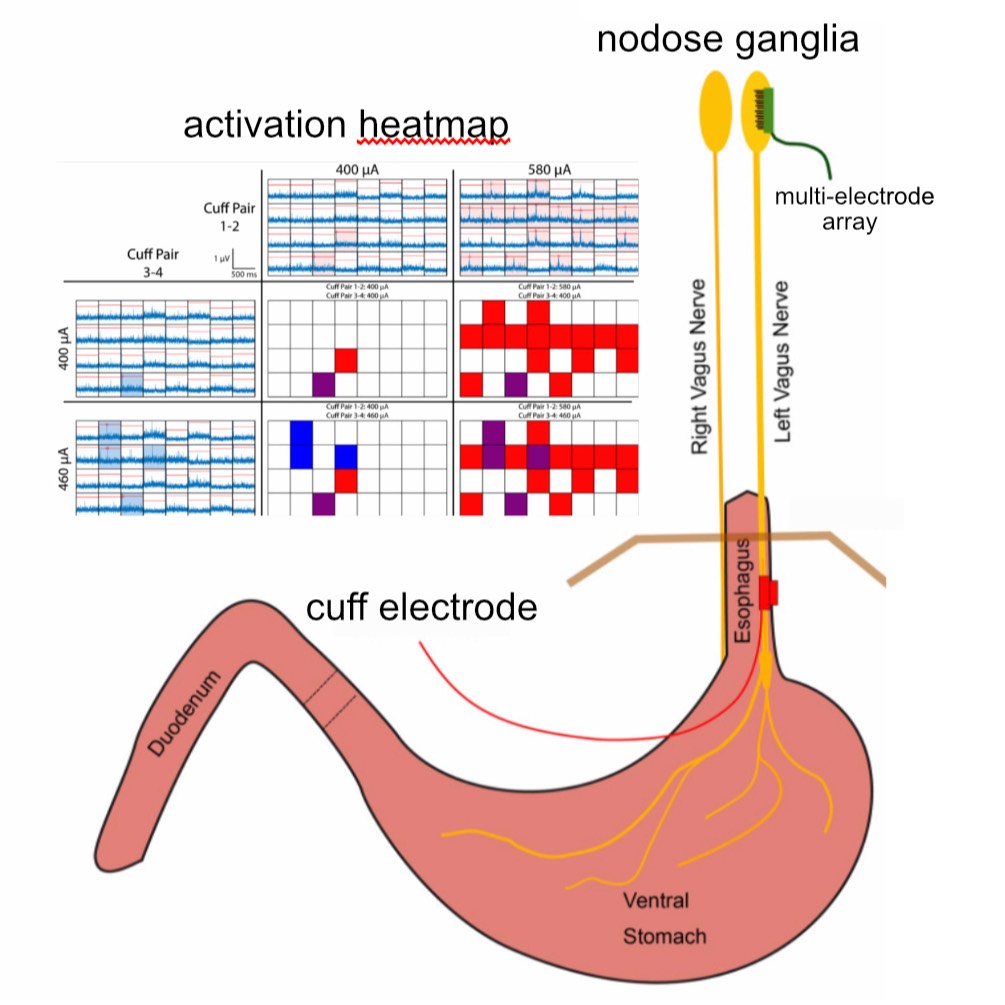
Dataset Overview
Study Purpose: In this study, we aimed to achieve selective stimulation of populations of vagal afferents using a multi-contact cuff electrode wrapped around the abdominal trunks of the vagus nerve.
Data Collection: Four-contact nerve cuff electrodes were implanted around the dorsal (N = 3) or ventral (N = 3) abdominal vagus nerve in six ferrets, and the response to stimulation was measured via a 32-channel microelectrode array (MEA) inserted into the left or right nodose ganglion. Selectivity was characterized by the ability to evoke responses in MEA channels through one bipolar pair of cuff contacts but not through the other bipolar pair. In the second group of ferrets (N = 3), we implanted cuff electrodes for stimulation and gastrointestinal planar electrodes for recording myoelectric responses to stimulation. The dataset contains both nodose ganglion and gastrointestinal myoelectric recordings.
Primary Conclusion: We demonstrated that it was possible to selectively activate subpopulations of vagal neurons using abdominal VNS. Additionally, we quantified the conduction velocity of evoked responses to determine what types of nerve fibers (i.e., Aδ vs. C) responded to stimulation. We also quantified the spatial organization of evoked responses in the nodose MEA to determine if there is somatotopic organization of the neurons in that ganglion. Finally, we demonstrated in a separate set of three ferrets that stimulation of the abdominal vagus via a four-contact cuff could selectively alter gastric myoelectric activity, suggesting that abdominal VNS can potentially be used to control GI function.
Curator's Notes
Experimental Design: All experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and were performed following ARRIVE and other relevant guidelines and regulations. Anesthesia was induced and maintained with inhaled isoflurane (5% induction, 1–3% maintenance). In six of the ferrets, a laparotomy was performed, followed by implantation of a four-contact nerve-cuff electrode (Micro Leads, Inc. Somerville, MA) around the ventral (n = 3) or the dorsal (n = 3) abdominal vagus trunk. The cuff electrode contacts (area: 0.5 mm2, spaced 1 mm × 0.6 mm, 600 µm inner diameter) were arranged in two bipolar pairs with electrodes in each pair offset from each other by 90°, and the pairs spaced equally around the circumference of the nerve to provide current steering for targeted stimulation. A 32-channel MEA (4-by-8 electrode arrangement with 400 µm inter-electrode pitch, 1 mm long shanks; Black-Rock Microsystems, Salt Lake City, UT) was implanted into the nodose ganglion with a pneumatic inserter for rapid insertion through the epineurium. Recordings of spontaneous single-unit activity were used to verify the insertion of the MEA into the nodose, and if necessary, additional impacts were applied to insert the device further. A platinum wire was placed near the nodose to act as a reference, and another platinum wire was inserted under the skin as the recording ground. In the additional three ferrets, an identical procedure was used to implant a four-contact nerve-cuff electrode around the ventral abdominal vagus nerve, and four planar electrodes, each with four contacts (Micro Leads, Inc. Somerville, MA), were sutured to the ventral gastric surface. A Grapevine Neural Interface Processor (Ripple, Salt Lake City, UT) and stimulation headstage (Nano2 + Stim) were used to deliver stimulation to the pairs of electrodes on the abdominal cuff, while a recording headstage (Nano2) was used to record evoked CAP signals from the nodose MEA and GI myoelectric activity from the serosal surface of the stomach. Nodose recordings were sampled at 30 kHz and filtered with a high pass filter at 150 Hz and low pass filter at 7500 Hz. A notch filter from 50 to 70 Hz was also applied to eliminate line noise. GI myoelectric signals were sampled at 30 kHz with a high pass filter at 0.1 Hz and a low pass filter at 7500 Hz.
Completeness: this dataset is a part of a larger study, "GI vagal pathways for emesis and feeding."
Subjects & Samples: Nine adult male ferrets (weight: 1–1.7 kg; Marshall BioResources, North Rose, NY, USA) were used in this study.
Primary vs derivative data: The primary data is organized in folders by subject ID. Each subject folder contains metadata.json file with a header containing all of the metadata associated with each animal, including the experiment day, number of stim trials, stim amplitudes, pulse widths, etc. Stimulation data recordings are stored in run subfolders. There is no derivative data folder.
Code Availability: A repository for analysis code with selective stimulation available at https://github.com/cchorn/SPARC_vagus_stim_optimization
Files
1 - 0 of 0 files
About this dataset
Publishing history
Cite this dataset
Tags
References
Is Source of
Is Supplemented by
C Horn, C., M. Miller, D., Fulton, S., J. Yates, B., E. Fisher, L., & C. Nanivadekar, A. (2019). SPARC - Acute surgery and experimentation of the gastrointestinal tract and vagus nerve in the ferret v1. https://doi.org/10.17504/protocols.io.6a7hahn
C Horn, C., M. Miller, D., Fulton, S., J. Yates, B., E. Fisher, L., & C. Nanivadekar, A. (2019). SPARC - Gastrointestinal myoelectric recordings from the behaving ferret v1. https://doi.org/10.17504/protocols.io.6a8hahw
C Horn, C., M. Miller, D., Fulton, S., J. Yates, B., E. Fisher, L., & C. Nanivadekar, A. (2019). SPARC - Chronic implantation of gastrointestinal and vagus nerve electrodes in the ferret v1. https://doi.org/10.17504/protocols.io.6crhav6
Described by
Nanivadekar, A. C., Miller, D. M., Fulton, S., Wong, L., Ogren, J., Chitnis, G., McLaughlin, B., Zhai, S., Fisher, L. E., Yates, B. J., & Horn, C. C. (2019). Machine learning prediction of emesis and gastrointestinal state in ferrets. PLOS ONE, 14(10), e0223279. https://doi.org/10.1371/journal.pone.0223279
